ANTIBODY TEST CLINICAL PERFORMANCE
This fact sheet contains information to help you understand the risks and benefits of using the COVID-19 IgG testing provided by EDP Biotech Corporation.
What is the Clinical Performance of this test?
EDP Biotech’s CLIA laboratory currently utilizes the serological assay developed by trusted partner Quansys Biosciences in Logan, Utah. This test is FDA EUA authorized, EUA200977. The results from their Clinical Agreement Validation N=576 sample submission are shown below:
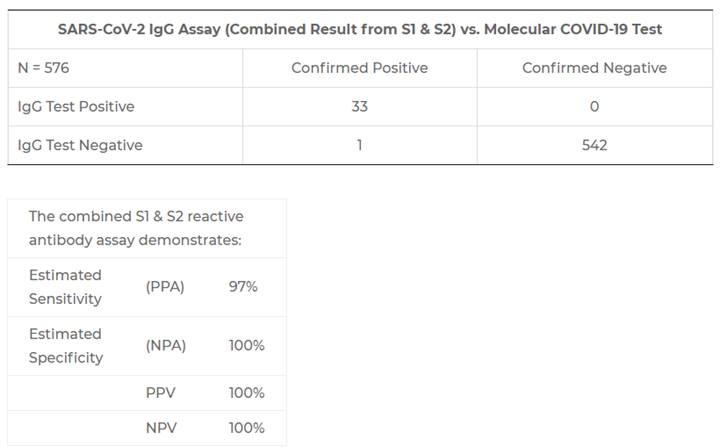
In-House Testing Results
Results of EDP Biotech’s in-house validation studies are shown below, confirming the Quansys Biosciences test performance in our Knoxville CLIA laboratory:
Clinical Agreement Study
The comparator method used to establish clinical truth is the Abbott RealTime SARS-CoV-2 RT-PCR test or samples collected prior to November 2019.
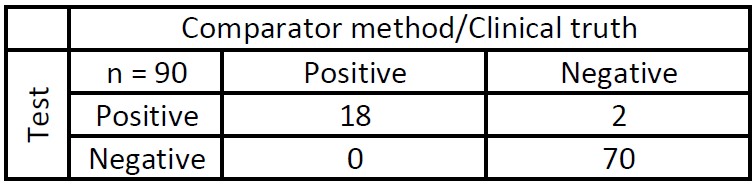
Sensitivity (Positive Predictive Agreement) 100%
Specificity (Negative Predictive Agreement) 97%
Acceptance Criteria PASSED
Reproducibility
Five samples tested on five separate days for agreement.
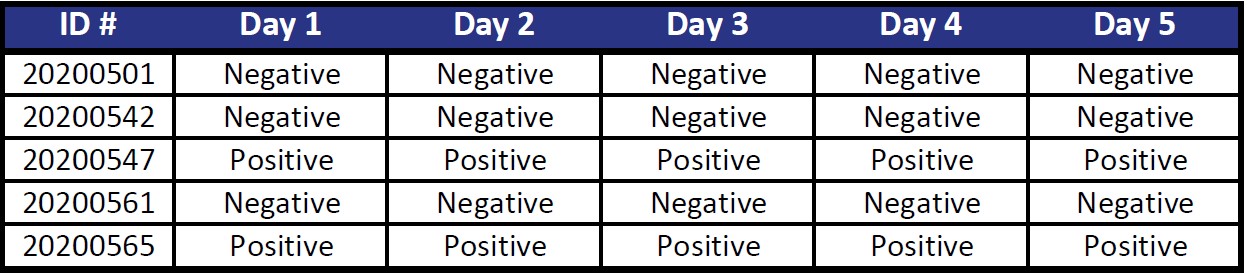
Percent Agreement 100%
Acceptance Criteria PASSED (Agreement >= 95%)
Cross-Reactivity
Five samples collected prior to November 2019 from each of the following disease states were tested for cross-reactivity to SARS-CoV-2 spike 1 (S1) and spike 2 (S2) antigens. Results are only considered positive when reactive to both S1 and S2.
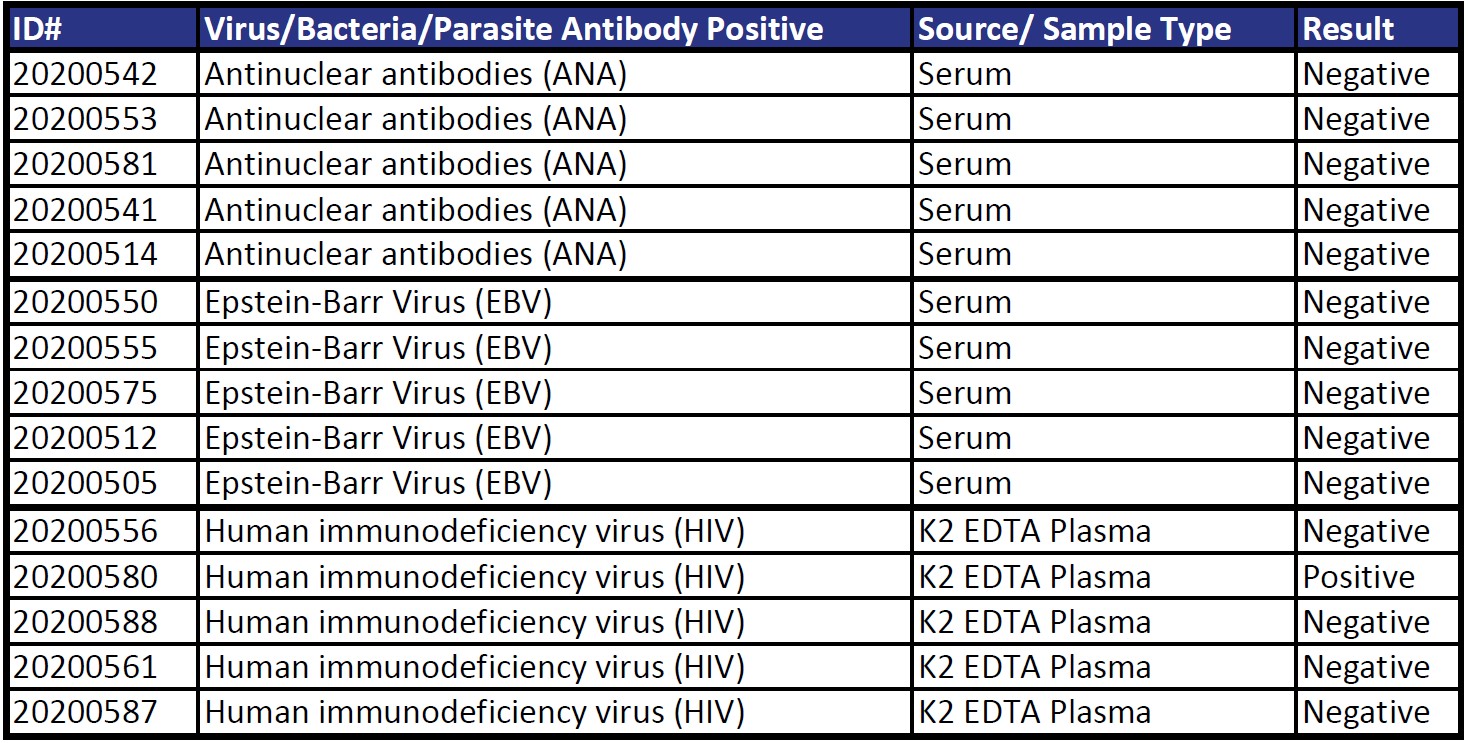
Total Percent Agreement (n = 90) 98%
Acceptance Criteria PASSED
Matrix Equivalency
Each possible matrix type collected at the same time from ten individuals and tested for agreement.
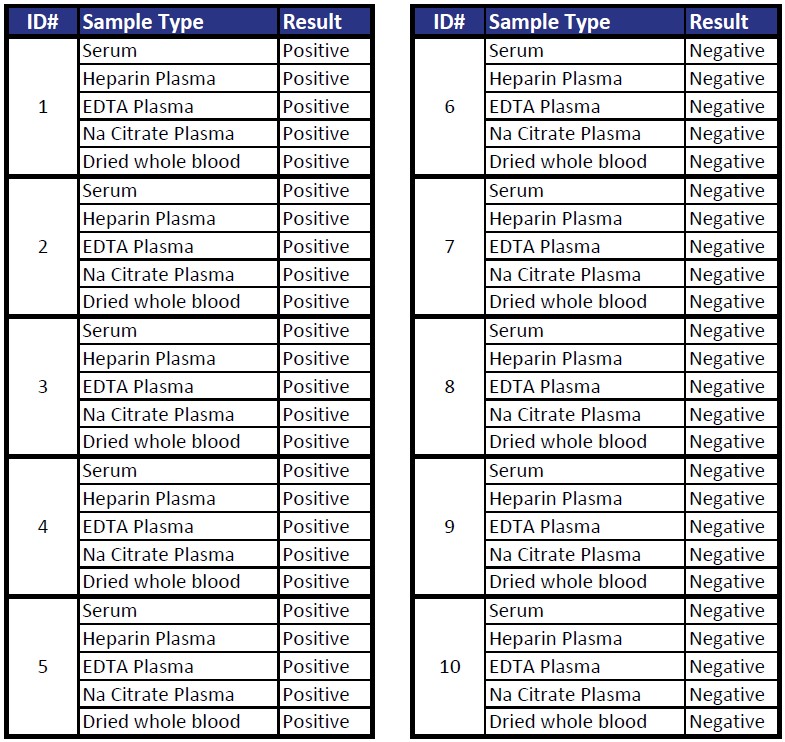
Percent Agreement 100%
Acceptance Criteria PASSED
